Network of University Medicine
Implementation concept for the establishment of a COVID-19 Data Exchange Plattform (CODEX) & National Research Network Applied Surveillance and Testing (B-FAST)
The current situation requires rapid action to contain the advancing pandemic. With the help of the data platforms of the Medical Informatics Initiative (MII), routine data from the clinical environment should be made effectively available. The demonstrator study on co-morbidities and rare diseases could demonstrate that the MII data integration centers currently under construction are already capable of quickly providing new, profitable insights.
Cooperation project with the MII:
Architecture, project organization and time planning
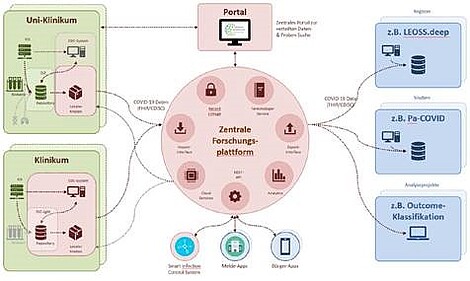
The architecture of the National Data Platform (see figure) is divided into decentralized parts ('NUM nodes' operated by the data integration centers) and a central component that supports data provision and use. In the first stage of expansion, the central platform will be ready for use within two months of the project start. It will be complemented by a central portal for data and biomaterial search. The provision of data relevant for COVID-19 will be made by the decentralized components to the central platform, but also by the partly already established decentralized components directly to national study registries.
The decentralized part of the platform ('NUM-node') uses existing infrastructures of the MII, especially the data integration centers that were established at almost all German university hospitals during the current funding phase of the MII (2018-2021). The data integration centers create the organizational and technical framework for the provision of data from primary clinical systems and also provide the technical possibility to integrate new components and processes (e.g. local COVID registers and SmICS at the respective university hospitals. With the technical infrastructure to implement the National Broad Consent of MII and to support local Use and Access Committees, they represent an essential building block for legally compliant and controlled secondary use of existing data. The decentralized components of the platform are also essential for the rapid and coordinated rollout of new guidelines, documentation standards and analytical tools throughout Germany.
The components of the platform at the respective locations are supplemented by a central platform with its own data stock, which enables joint research on the data integrated there and support of supply processes in the COVID-19 context. The central database contains pseudonymized data from various sources, especially the data integration centers, potentially also from health authorities and data provided directly by citizens (e.g. from apps). In operation, the platform will enable the large-scale roll-out of new services for providers, health authorities and citizens. On the one hand, this will enable efficient support for public health measures and, on the other hand, it will provide the data basis for making essential decisions in dealing with the COVID 19 crisis. The uniform platform consisting of central components and local nodes makes it possible to support such decision-making processes quickly and in a qualified manner with a reliable data basis and to offer diagnostics and therapies tailored to individual patients throughout the entire network.
Project Team
M.Sc. C. Drenkhahn
M.Sc. J. Wiedekopf
M.Sc. A.-K. Kock-Schoppenhauer (ITCR-L)
Dr. B. Kroll (ITCR-L)
Prof. Dr. J. Ingenerf

- Research
- AI und Deep Learning in Medicine
- Medical Image Processing and VR-Simulation
- Integration and Utilisation of Medical Data
- Sensor Data Analysis for Assistive Health Technologies
- Medical Image Computing and Artificial Intelligence
- Medical Data Science Lab
- Medical Deep Learning Lab
- Junior Research Group Diagnostics and Research of Movement Disorders
- Former Medical Data Engineering Lab
Contact person
Josef Ingenerf

Retired Professor
josef.ingenerf(at)uni-luebeck.de