Semantic HL7 Annotations of Clinical Device Data for the Integration of Smart Applications
Information processing in health care is characterized by distributed and mainly insufficiently integrated information systems. Within a hospital, patient data is exchanged using the communication standard HL7 version 2 for the major, often reimbursement-related tasks. In specialty areas such as operating rooms or intensive care, however, there is a lot to be done. The main problem is the lack of integration of the numerous medical devices and their proprietary device interfaces.
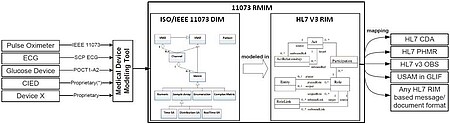
For quite some time the ISO/IEEE 11073 standard exists for implementing a vendor-independent device data exchange. Essentially it describes standardized data structures (classes, properties, values, units) for the respective medical devices and messaging protocols to exchange data itself. This project with Dräger is less about the data exchange with devices, as relevant patient and device data are already stored within in the intensive care information system "Integrated Care Manager" (ICM). The data, however, should be mapped into an IEEE 11073 compliant data format so that the data can be processed reliably. Only then it can, for example, also be used by Draeger for integrating "SmartCare"; a decision support system for automatic weaning mechanically ventilated patients that should be applied to different customers without costly customization.
An interface for Smart Care must provide a defined set of patient and device data from the ICM-system in a suitable format. For maximum flexibility device data is not directly mapped to IEEE 11073 - compliant formats via a Domain Information Model (DIM), but indirectly integrated in a suitable HL7 v3 RMIM (Refined Message Information Model) called “11073 RMIM”. On this basis, desired output formats can be derived generally through XSL transformations, e.g. the CDA-based PHMR (Personal Healthcare Monitoring Report) specifically created for exchanging device data.
In course of the project, a mapping tool will be developed with the following stages:
Once only:
- Adaptation of the generic 11073 RMIM model for more than one device class and for several patient data.
Initialization:
- for each new device: Provision or adaption of IEEE 11073 DIM schemes for device classes of interest and initially a partial instantiation of the 11073 RMIM.
- for any new intensive care information system: Provision or adaption of a simple XML-based intermediate format, including a setup routine for mapping proprietary device codes in the IEEE 11073 standard.
- for each new output format: Provision or adaption of XSL transformation rules for mapping a 11073 RMIM instance to the desired output format, e.g. for a PHMR document.
At run time:
- V3MapCore: The exported ICM-data are mapped to the standard output format after instantiation of the adapted 11073 RMIM.
This mapping tool makes it possible to mediate patient and proprietary device data in a way that further processing applications like the SmartCare system can use the data (Plug & Play). An adaption to other devices, intensive care information systems and output formats can be achieved after performing the initialization steps mentioned above [2]. Due to the increasing importance of the SOA architecture for integrating devices or information systems it is intended to provide HL7 V3-conformant payloads for web services as further output format instead of a static PHMR documents.
Selected Publications
- Yuksel M, Dogac A. Interoperability of Medical Device Information and the Clinical Applications: An HL7 RMIM based on the ISO/IEEE 11073 DIM. IEEE Trans Inf Technol Biomed 2011; 15 (4): 557-66. (LINK)
- Zeplin G, Kock A-K, Poelker M, Seidl K, Mersmann S, Ingenerf J. Semantische HL7v3 Annotation klinischer Messwerte aus einem PDMS mittels ISO/IEEE 11073 Gerätebeschreibungen zur Anbindung von Smart Applications, 2011. (LINK)
- Josef Ingenerf, Ann-Kristin Kock, Marcel Poelker, Konrad Seidl, Georg Zeplin, Stefan Mersmann, Heinz Handels. Standardizing Intensive Care Device Data to Enable Secondary Usages. Stud Health Technol Inform. 2012;180:619-23. (LINK)
- Ann-Kristin Kock, Josef Ingenerf, Stoyan Halkaliev, Heinz Handels. Migration Path for Structured Documentation Systems including Standardized Medical Device Data. Stud Health Technol Inform. 2012;180:43-7. (LINK)
Projekt Team
M. Sc. Ann-Kristin Kock (ITCR-L)
B. Sc. Marcel Poelker (Student)
B. Sc. Konrad Seidl (Student)
B. Sc. Georg Zeplin (Student)
PD Dr. rer. nat. Josef Ingenerf
Cooperation Partners
Stefan Mersmann, Dräger Medical GmbH, Lübeck, Germany

- Research
- AI und Deep Learning in Medicine
- Medical Image Processing and VR-Simulation
- Integration and Utilisation of Medical Data
- Sensor Data Analysis for Assistive Health Technologies
- Medical Image Computing and Artificial Intelligence
- Medical Data Science Lab
- Medical Deep Learning Lab
- Medical Data Engineering Lab
- Junior Research Group Diagnostics and Research of Movement Disorders
Contact person
Josef Ingenerf

Professor
Gebäude 64, 2nd floor
,
Raum 11
josef.ingenerf(at)uni-luebeck.de
+49 451 3101 5625